Avner Australian Pancreatic Cancer Matrix Atlas (APMA)
The Avner Australian Pancreatic Cancer Matrix Atlas (APMA) is a national and international portal for pancreatic cancer and matrix biology research.
About APMA
The Avner Australian Pancreatic Cancer Matrix Atlas (APMA) is a national and international portal for pancreatic cancer and matrix biology research. Desmoplasia (fibrosis) is a key hallmark of pancreatic cancer, which can be targeted in concert with tumour cell-centric therapies. The overarching goal of APMA is to facilitate the translation of stromal-centric therapy as a mainstay in our emerging multimodal armoury against pancreatic cancer. We have a number of ongoing extracellular matrix (ECM) drug targeting programs where we can rapidly test matrix manipulations from this resource (with or without) standard-of-care chemotherapy.
Approach
Using novel tissue analysis and decellularisation techniques, we map the distribution, organisation, and assembly of fibrotic stroma in human pancreatic cancer and matched non-malignant adjacent tissue to identify new ECM targets specific to pancreatic cancer progression.
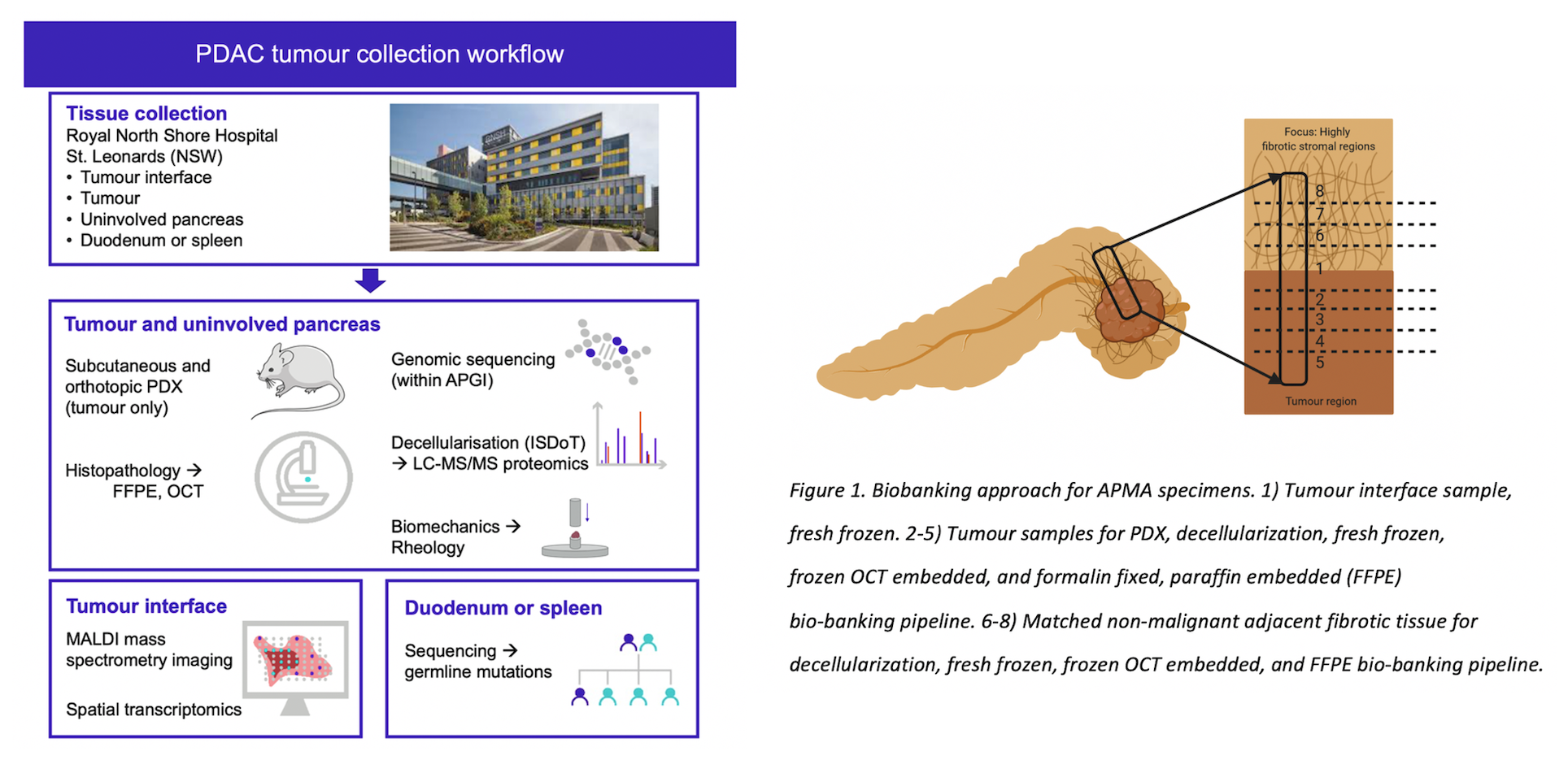
Samples are collected fresh from surgery from tumour regions and matched non-malignant adjacent fibrotic tissue for each patient. Each tissue sample is split into multiple parts and bio-banked as either: i) fresh frozen, ii) frozen OCT embedded, iii) formalin fixed, paraffin embedded, iv) xenografted orthotopically or subcutaneously in immunocompromised mice, or v) entered immediately into our up-and-running decellularisation pipeline (Figure 1). Specimens are then fully annotated and subsequently sequenced. Notably, we are the first team to have optimised an innovative decellularisation approach for the assessment of matrisomal proteins in human pancreatic tissue specimens. This comprehensive biobanking methodology aligns with the APGI core mission and provides a multimodal approach to targeting pancreatic cancer from either the epithelial or stromal compartment. Overall, APMA provides a world class resource from which to base novel targeted therapies in the era of personalised medicine and microenvironmental influence in this aggressive disease.
Vision and goals
We aim to better understand how the dynamic ECM landscape is linked to alterations in drug response and patient survival. By mapping the histological, immunohistochemical, and proteomic differences between normal fibrotic pancreatic tissue and matched pancreatic cancer we aim to define which ECM features are deregulated in this disease and reveal new ECM targets. Detailed comprehensive mapping of the topology and 3D structure of the ECM in native pancreatic tissue and pancreatic cancer, in correlation with clinicopathological and genetic sequencing data, has not been possible until now. The Garvan Institute of Medical Research, working with the Royal North Shore Hospital (RNSH) here in Australia are perfectly placed to achieve this, thereby establishing the first national and international comprehensive human pancreatic cancer ECM database.
APMA is an international resource held in Australia for pancreatic cancer matrix targeting, creating an international portal for matrix biology researchers and clinicians to interrogate and query disease progression, outcome, survival and relapse in the context of ECM composition and 3D matrix topology. Working with the clinical team at RNSH we will integrate our ECM findings with clinicopathological data including survival, relapse, and resistance, to identify ECM companion biomarkers and actionable targets that may be diagnostic and/or prognostic.
Executive Leadership Team

Prof. Paul Timpson (Co-founder)
Paul is Laboratory Head of the Garvan’s Invasion and Metastasis group, focusing specifically on imaging live pancreatic tumour behaviour in the context of disease progression and improving drug targeting. This live imaging work has now been correlated to TMA patient biopsy data from the APGI cohort helping translation of the groups’ findings.

A/Prof. Thomas Cox (Co-founder)
Thomas currently leads the Matrix and Metastasis Lab at the Garvan Institute of Medical Research within The Kinghorn Cancer Centre. The labs creative research program integrates matrix biology with precision oncology, under the strategic vision to make fundamental advances in personalised stromal targeting in solid tumours. Leveraging our decellularisation platforms alongside our global and spatial proteomic approaches, we are helping map the distribution, organisation, and assembly of the extracellular matrix in the APMA cohort.

A/Prof. Marina Pajic
Marina Pajic is a translational cancer researcher, Head of the Personalised Cancer Therapeutics Laboratory at the Garvan Institute. Her research in pancreatic cancer over the past 10 years has led to major improvements in our understanding of the genetics of pancreatic cancer. Her work’s focus is on using this critical information to identify new personalised treatment approaches for pancreatic cancer and she has been a long-standing critical player in the Australian Pancreatic Genome Initiative (APGI) based at Garvan.

Prof. Anthony Gill
Anthony is Professor of Surgical Pathology at the University of Sydney and chairman of the APGI. He graduated in medicine from the University of Sydney in 1997 with the Harry J Clayton Memorial Prize for first in university in Medicine and Clinical Medicine; the Hinder Memorial Prize for first in the university in Surgery and Clinical Surgery; the Robert Scot Skirving Memorial Prize for first in the university in Medicine and Surgery and the Royal North Shore Hospital Medal. He completed his specialty training in surgical pathology (FRCPA) in 2005 and since then has divided his time between diagnostic pathology as a senior staff specialist at Royal North Shore Hospital and medical research where he concentrates on translating advances made in the understanding of cancer at the basic science or molecular level into diagnostic tests which can be applied in the routine surgical pathology laboratory on patient biopsy specimens. He has co-authored more than 500 original research publications and his research has been recognized with numerous national and international awards including both the Benjamin Castleman Award and the Ramzi Cotran Young Investigator award from the United States and Canadian Academy of Pathology, the Sir John Lowenthal Medal, the NSW Premier’s Award for Excellence in Translational Cancer Research, the Sir Zelman Cowen Universities Prize for discovery in Medical Research and the Distinguished Pathologist Award presented by the International Academy of Pathologist (IAP). In 2018 he was appointed Member of the Order of Australia (AM) “for significant service to medical research in the field of surgical pathology as an academic, author; adviser, and mentor.

Dr. David Herrmann
David is a Senior Postdoctoral Scientist in the Invasion and Metastasis group at the Garvan Institute of Medical Research. David combines subcellular intravital imaging technologies and biosensor models to quantify and track dynamic, ‘difficult-to-assess’ aspects of cancer progression, metastasis and response to treatment in the live tumour microenvironment. Our launch of the APMA cohort of fibrosis-enriched patient samples will provide a unique resource for pre-clinical studies of pancreatic cancer, which is known display high tissue fibrosis, and help validate novel anti-fibrotic therapies in combination with standard-of-care in this deadly disease.

Dr. Brooke Pereira
Brooke is a post-doctoral research officer and Cancer Institute NSW Fellow in the Cancer Invasion and Metastasis group at Garvan. She uses cutting-edge approaches such as bioengineering, proteomics, genomics, patient-derived xenografts (PDX), spatial proteomics (MALDI mass spectrometry imaging), spatial transcriptomics & intravital (in vivo) imaging to investigate novel anti-tumour agents in pancreatic cancer. Her research focuses on the dynamic role of stromal components of the tumour microenvironment such as cancer-associated fibroblasts (CAFs), infiltrating immune cells and the extracellular matrix (ECM) in invasion and metastasis.

Dr. Shona Ritchie
Shona is a Postdoctoral Research Officer in the Invasion and Metastasis group at the Garvan Institute. Shona investigates approaches to overcome therapeutic challenges in the treatment of metastatic pancreatic cancer. Her expertise encompasses multi-omics analysis, advanced in vivo techniques, intravital imaging and the development of patient-derived models, to enhance the translation of her research, in order improve outcomes for pancreatic cancer patients.

Prof. Jas Samra
Professor Jas Samra is a Clinical Professor of Surgery at the University of Sydney. He graduated from medicine and did his training in the United Kingdom, which included a three-year Welcome research fellow at Oxford University resulting in a degree in Doctor of Philosophy. This has led to his lifelong interest in basic sciences in surgery.
On moving to Australia, he did his surgical training at Royal North Shore Hospital. For the last seventeen years he has been a surgeon in Northern Sydney at Royal North Shore Hospital as well as Ryde Hospital. He has been a key clinical member of the Australian Pancreatic Genome Initiative. He has a keen interest in integration of innovation and new technology in clinical practice. He has been a key member in setting up services for pancreatic cancer and pancreatic neuroendocrine tumours at Royal North Shore Hospital and North Shore Private Hospital in the last decade. He has presented the Unit’s work both nationally and internationally and continues to be active in basic and clinical research.
Professor Samra was the first da Vinci accredited robotic general surgeon in Australia as well as the first surgeon in NSW to perform robotic distal pancreatectomy and liver resection
Professor Samra was also awarded the Medal of the Order of Australia, for his efforts, significant contribution into research in pancreatic cancer and his service to medicine as a pancreatic specialist in 2019.

Amber Johns
Amber’s research experience has focused on establishing and coordinating the development of complex, novel, large-scale research projects. Amber has clinical experience in pathology and cytology, and has been instrumental in building the Australian Pancreatic Genome Initiative (APGI) as part of Australia’s contribution to the International Cancer Genome Consortium.
Through the APGI, Amber developed and coordinated a national pancreatic cancer biobank network, with international linkage. Amber has the unique blend of clinical, community and scientific experience, which she has successfully applied in various settings with a range of stakeholders. Her unique skill set has been pivotal in the success of not only her own local groups, but providing a platform for cancer research groups across the world to thrive, through her ability to be the link between the research team, the clinical team, and patient and community groups.
Amber has extensive experience in ethical, policy and societal issues in research and currently advises the Federal Government in relation to genomic sequencing and the return of research results.
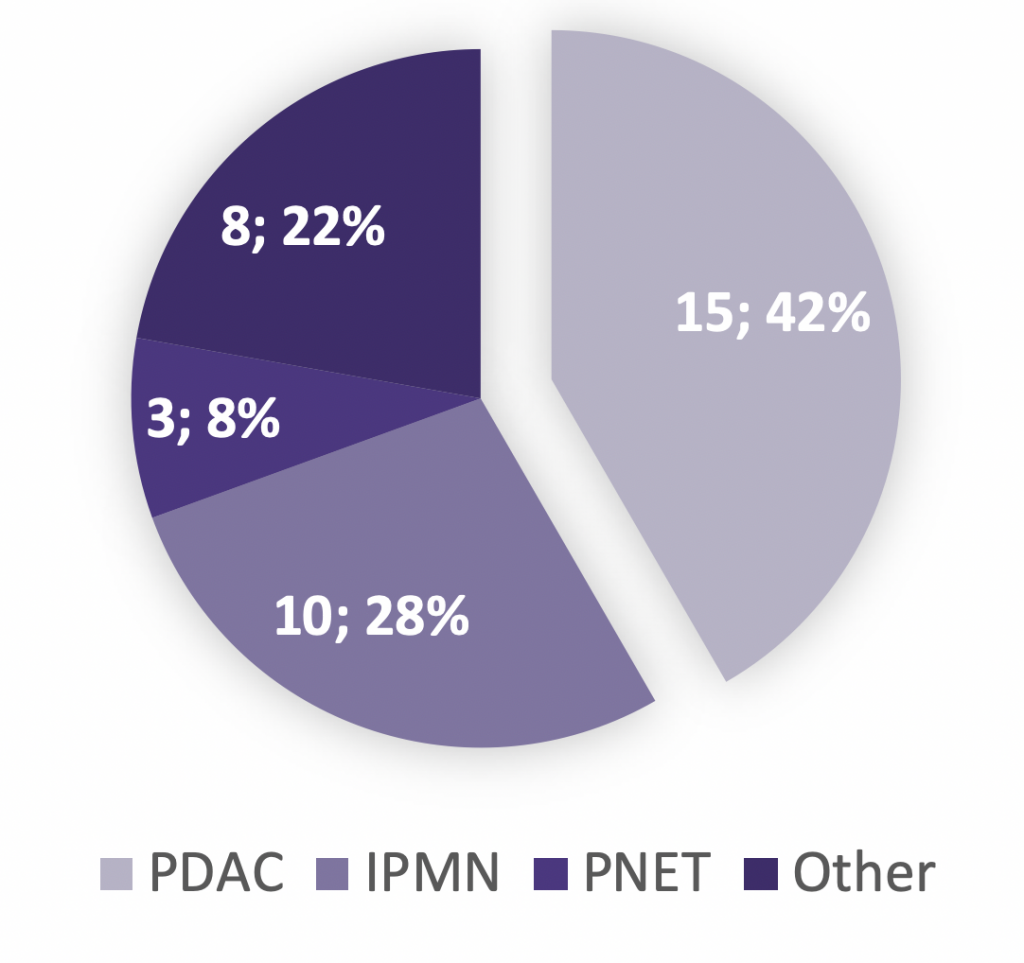
Patient enrolment to date
Fresh human specimen sets (pancreatic tumour, adjacent non-neoplastic pancreas, and duodenum or spleen) have been collected from Whipple’s procedures at RNSH. Patient recruitment and samples are being banked on a continual basis.
BioResource availability
All collections have snap-frozen specimens from the fresh primary pancreatic tumour, as well as a matched adjacent non-neoplastic pancreas specimen and a duodenal or splenic specimen, which are available upon request via the APGI BioResource Request Form. The primary pancreatic tumour and adjacent non-neoplastic pancreas specimens were also formalin-fixed paraffin-embedded (FFPE) and embedded and frozen in OCT. For PDAC tumours, orthotopic and subcutaneous PDXs are set up. Successful PDXs are then serially passaged, with cryopreserved specimens available upon request. The samples are sequenced enabling full integration with the APGI. All specimens are also available upon request using the APGI BioResource Request Form.
Contact Us
Avner Australian Pancreatic Cancer Matrix Atlas (APMA)