.
.
.
.
Published earlier this month, a study led by APGI researchers has provided the most in-depth analysis yet of pancreatic cancer, re-classifying this disease into four subtypes, each with its own genetic make-up and distinct behaviour. This gives us insight into why tumours seem to act differently in individual patients.
In this study, we performed extensive genetic analyses of 456 pancreatic patients, identifying trends of recurring mutations throughout their genes. Some of the genes identified were known previously to function abnormally in pancreatic or other cancers, but we also discovered many mutations in genes with no previous association with cancer, opening up exciting new avenues for therapeutic exploration.
2,500 Australians die from pancreatic cancer every year
Currently 3,000 new pancreatic cancer cases are diagnosed in Australia each year, and this number has been increasing gradually since the 1980s. Medical developments have seen survival rates for patients with other cancers improve significantly over the last three decades; for example breast cancer patients now have a 90% chance of surviving five years after diagnosis. If current trends continue, pancreatic cancer is predicted in America to become the second-highest cause of cancer death within ten years, and this is likely to be mirrored here in Australia.
We need to use our scientific knowledge to change this. In general, chemotherapy used in pancreatic cancer is not very selective; nearly all patients receive similar treatment despite increasing scientific evidence showing how genetically diverse pancreatic tumours are – to quote the scientists themselves, it’s like hitting the disease with a mallet with your eyes closed.
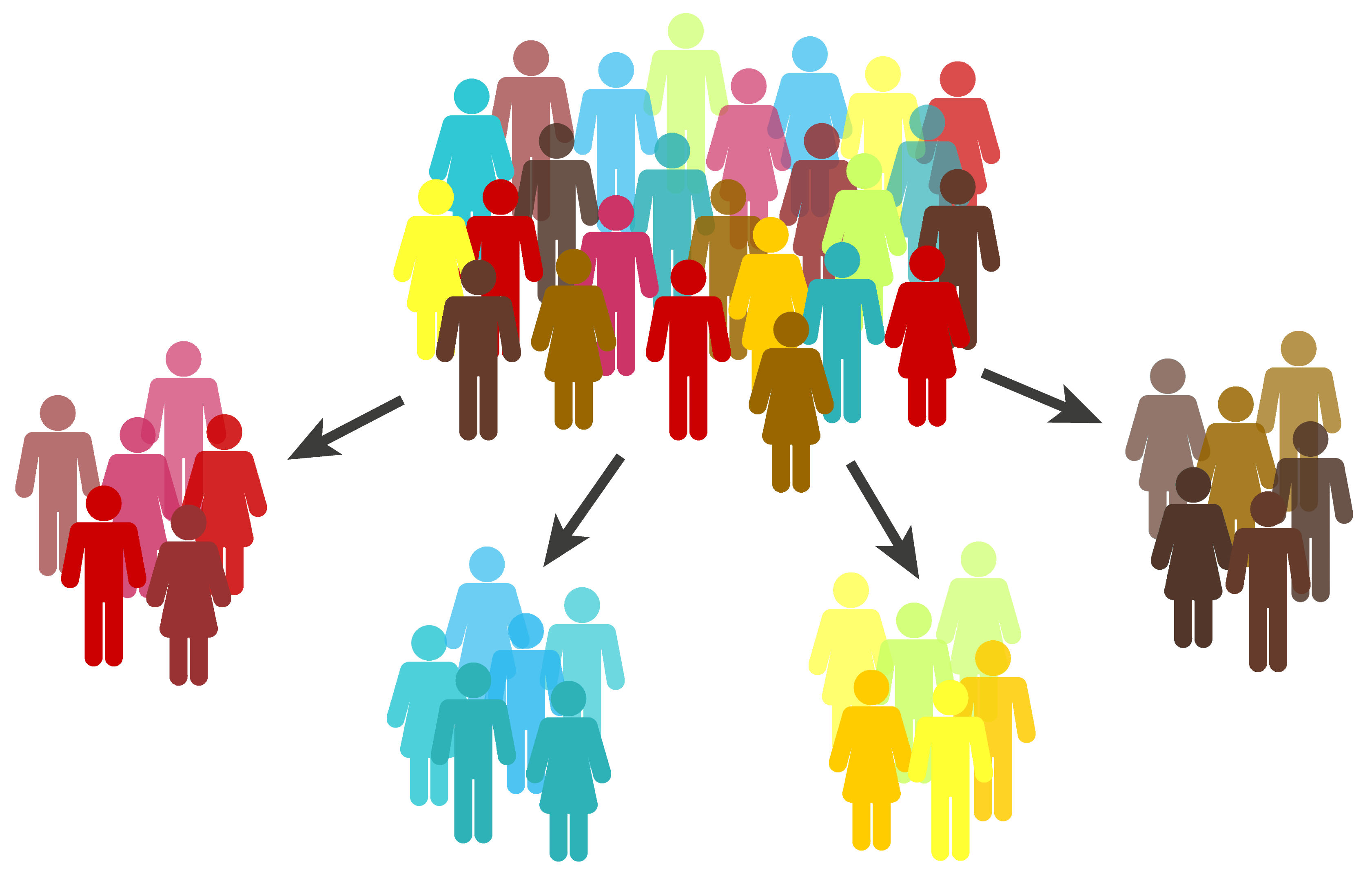
Pancreatic cancer is more than one disease
The re-classification of pancreatic cancer into four different diseases is a major step forward. Our researchers examined the whole genomes (the complete genetic make-up) of tumours, revealing the most comprehensive picture yet of the genomic landscape of pancreatic cancer. We then went one step further, examining the overall gene expression profiles – this type of analysis gives valuable information into which genes are turned on or off, or expressed. When we looked at all this data together, it was clear that pancreatic tumours could be grouped together into four different subtypes with very distinct sets of characteristics:
Squamous subtype
This subtype has genetic characteristics typically observed in squamous-type cancers of the breast, bladder, lung, and head and neck cancers (squamous cells are thin, flat cells that line the surface of the skin and many of our internal organs). Unfortunately, this subtype was associated with shorter survival compared to the other groups.
Pancreatic progenitor subtype
These tumours have specific genetic profiles similar to cells in early pancreas development in the embryo. Embryonic gene programs are usually switched off when cells have matured; however, cancer cells often exhibit abnormal developmental gene activity.
Aberrantly differentiated endocrine exocrine (ADEX) subtype
This subclass of tumours has genetic networks typical of later stage pancreatic development.
Immunogenic subtype
Tumours of this subtype have markedly irregular activity of genes associated with immune suppression; increased activity of these genes may enable tumours to escape attack by the immune system.
Immunotherapy: using the immune system to fight cancer
One of the most exciting and almost immediately actionable findings of this study is the identification of the novel immunogenic subtype. Cancer immunotherapy, which increases the strength of the immune response against tumours, has generated particular interest in recent years due to success in clinical trials in melanoma, non-small cell lung cancer and some other cancers. One-quarter of pancreatic tumours were seen to have high expression of multiple genes involved in immunosuppression – two examples are CTLA4 and PD1, and blood tests to measure these genes have already been developed and targeted therapies (drugs that target specific genes) are available. Two such drugs, called Yervoy and Keytruda, are being used to fight melanoma and are currently in clinical trials for other cancers.
The discovery may be ground-breaking: but what does this mean for patients?
Profiling of cancers in this way has revolutionised the clinical management of other cancers in the last few decades. In the 1980s, scientists discovered that almost a quarter of breast tumours had increased levels of the gene HER2. This gene causes particularly aggressive cancer growth and was originally indicative of a very poor outcome. However, after the discovery of this gene in the laboratory, the drug Herceptin was developed, which specifically targets HER2, and the outcome for patients with HER2-positive breast cancer improved dramatically. Since the early 2000s, genomic profiling has transformed breast cancer management even further. Studies of hundreds of tumours categorised breast cancer into four different subtypes, one being HER2-positive, leading to the development of many more therapies tailored to specific groups of patients. Panels of biomarkers (genes that mark the presence of disease) from these studies have been developed into clinical tests for diagnosis of disease, for providing information about outcomes (prognostic information), and for predicting response to chemotherapy or targeted therapies. More effective combination of chemotherapy with targeted agents, or multiple therapies targeting different genes, has helped bring the five-year survival rate for people diagnosed with breast cancer from 72% in the 1980s to 90% today.
We have reason to hope that future progress with pancreatic cancer care will be speedier. A number of the genes identified in this study have been identified in other cancers and have been targeted by novel drugs in development or in the clinic already – for example, the cancer promoting gene (oncogene) MET, found mutated in this study, has been implicated in a number of cancers and several MET inhibitors are in clinical development. The squamous subtype identified here had elevated EGFR activity; EGFR also plays a role in several cancers, and EGFR inhibitors are already used to treat some types of lung, breast and other cancers.
The future: putting us one step ahead of the cancer
We now have a deeper understanding of pancreatic cancer biology than ever before. Laboratory research into the newly identified gene mutations is continuing all over the world in order to figure out how they can be exploited in the clinic. There may be the opportunity for some clinical trials to begin swiftly based on select subtypes identified in this work. Our major focus at the APGI is being able to use this information in real-time when patients are first diagnosed, to reveal more accurate, personalised information on outcome and response to therapy, and so that clinical intervention can begin before the cancer has spread. Putting us one step ahead of the cancer instead of behind. This is the goal.
This work was only made possible by the hundreds of pancreatic cancer patients who generously donated their time, energy, tissue samples and personal information to us. This invaluable resource is paving the way to a better future for pancreatic cancer patients.