Researchers at the Garvan Institute have developed a novel way to monitor drug response and resistance in pancreatic cancer, utilizing a mouse model that allows for the real time visualisation of the effect of drug treatment within tumours.
The team at the Garvan Institute co-lead by Professor Paul Timpson and Dr Max Nobis, developed a biosensor mouse model that produces a fluorescent version of the molecule AKT. AKT maintains normal growth and metabolism in health cells, however in many cancers, including a quarter of pancreatic cancers, an altered version of AKT is present which results in the growth and spread of cancer cells.
Using the mouse model developed by the team at the Garvan Institute, AKT activity and response to drug treatment can be monitored in real time within live tissue. The activity is visualised using intravital microscopy that provides real time footage of cellular behaviour within the live tissue.
‘Pockets’ of drug resistance
The overall 5-year survival rate of pancreatic cancer has remained unchanged for decades. Less than 11% of cases survive beyond 5 years, this rate drops below 3% once the cancer has metastasised. New developments in treatments are critically needed. The AKT pathway is a promising therapeutic target for treatment of the disease, unfortunately drugs targeting the AKT pathway alone have been largely clinically ineffective.
Professor Paul Timpson, co-senior author of the study, commented “Our biosensor mouse model is the first tool capable of showing us exactly when and where AKT switches on or off in the living tissues, providing us with an unprecedented view of its treatment response and resistance,”
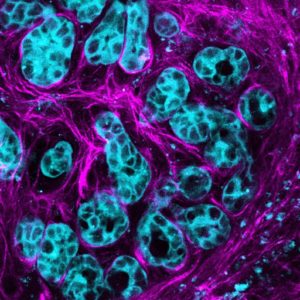
Pancreatic cancer cells activate the molecule AKT (blue) to multiply and spread along connective stroma (pink) in the cancer environment. Credit: Max Nobis (VIB-KU Leuven Center for Cancer Biology)
The team inhibited AKT activity with clinical therapies and observed that the AKT pathway remained active inside drug resistant ‘pockets’ situated within areas of the tumour with low oxygen supply or areas bordering the spread of cancer. According to Professor Timpson, there was also evidence to indicate that “AKT not only drives cancer growth but it also actively drives the early events of cancer spread to other sites around the body”.
Co-senior author, Dr Max Noblis further stated “Now that we can visualise pockets of treatment response and resistance, our next steps will be to investigate treatments co-targeting AKT in combination with drugs that open up blood vessels for improved drug delivery, while also breaking down known barriers such as dense fibrotic tissue that often surrounds pancreatic tumours. This could improve drug delivery and switch off AKT inside impenetrable pockets to help reduce drug resistance. Seeing such combination approaches in real-time inside a tumour will be crucial to improving treatments”
The novel biosensor mouse coupled with intravital imaging technology will continue to be used as a tool to investigate the effects of a combination of therapies in pancreatic cancer. Targeting treatments that mitigate drug resistance and reduce tumour growth and spread through the inhibition of the AKT pathway, provide an approach to improving the overall outcome of pancreatic cancer patients.
“Our ability to see AKT in cancer cells is taking the guesswork out of the development of new therapies and has broad applications, with AKT contributing to many cancers, including breast and prostate cancers. Our model is a vital new tool in the era of co-targeting the tumour ecosystem in precision medicine, bringing us closer to improved treatment options for patients,” says Professor Timpson.
The work was published in the journal Science Advances.
